본문
Infectious disease test HPV Genotyping 9G Membrane KIT™ (CE, KFDA)
- Sample type
- Vaginal or Urethral swab, Endo cervical swab, Urine
- Shelf life
- 12 months
- Package
- 48 tests/kit
- Compatible Device
- BMT 1D scanner, BMT 9G-1000 analyzer
HPV Genotyping 9G Membrane KITt™ detects Human Papilloma Virus(HPV) that causes cervical cancer.
· Genotyping 5 HPV subtypes (16, 18, 45, 31, 33) belonging to the high risk group.
· Screening 9 HPV subtypes (35, 39, 51, 56, 58, 59, 66, 68) belonging to the high risk group.
· HPV Genotyping 9G Membrane KITt™ detects HPV very sensitively(Limit of Detection : 1-100 copies/ml).
Technical Excellence in Biometrix Technology
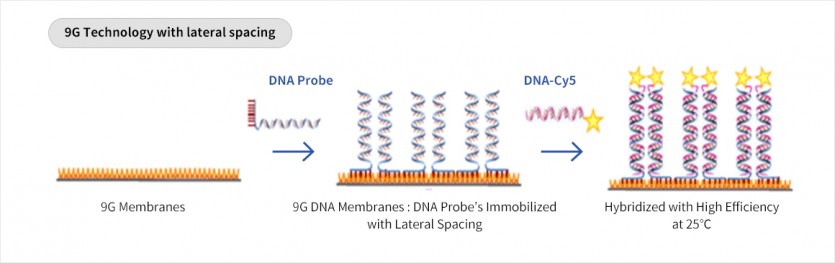
· Detection of trace amounts of DNA using 9G technology.
· Hybridization at room temperature is possible because of constant interval between DNA probes.
· Genotyping for pathogens is possible because SNP(Single nucleotide polymorphism) can be identified through DNA-DNA binding.
- References
-
- HPV 9G DNA Chip : 100% Clinical sensitivity and specificity. J Clin Microbiol. 2012
- Comparison of the cobas 4800 HPV and HPV 9G DNA chip tests for detection of high-risk human papillomavirus in Cervical specimens of women with consecutive positive HPV tests but negative pap smears. PLoS ONE. 2015
- HPV genotyping 9G membrane test : A point of care diagnostic platform. Sensors. 2014
- Prev9G test™ ultra Troponin T & NT-proBNP (CE, KFDA) 19.08.03
- Next6 STD Genotyping 9G test™ 19.08.03