본문
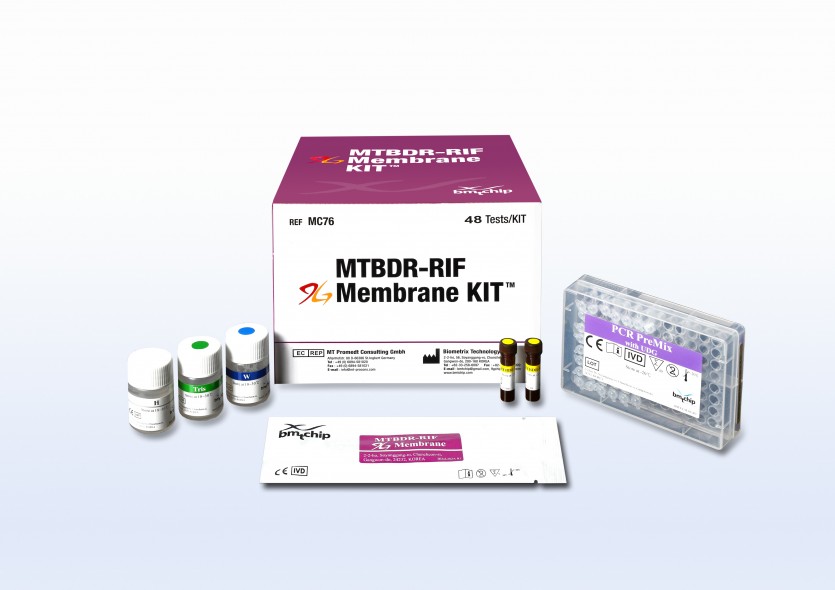
Infectious disease test MTBDR-RIF 9G membrane kit™ (CE, KFDA)
- Sample type
- Sputum
- Shelf life
- 12 months
- Package
- 48 tests/kit
- Compatible Device
- BMT 1D scanner, BMT 9G-1000
Specification
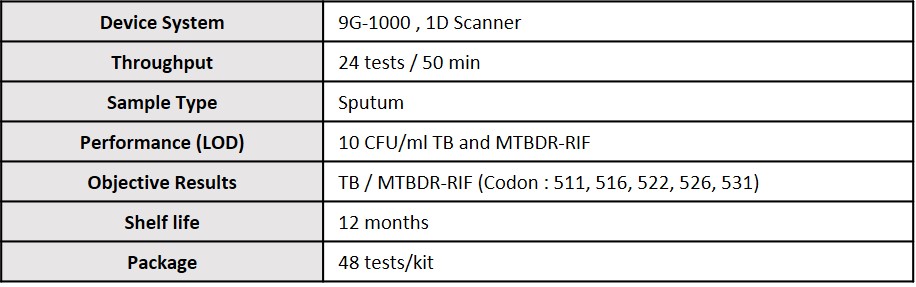
Diagnosing Tuberculosis and MTBDR-RIF with a single PCR.
· Detection of M.Tuberculosis and the identification of rpoB gene mutations (531, 526, 522, 516, 511) that induce drug resistance to Rifampin.
· MTBDR-RIF 9G membrane KIT™ detects M.Tuberculosis very sensitively (Limit of Detection : 10 CFU/ml)
· The World's only molecular diagnostics product capable of simultaneously detecting TB and MTBDR-RIF when monitoring treatment.
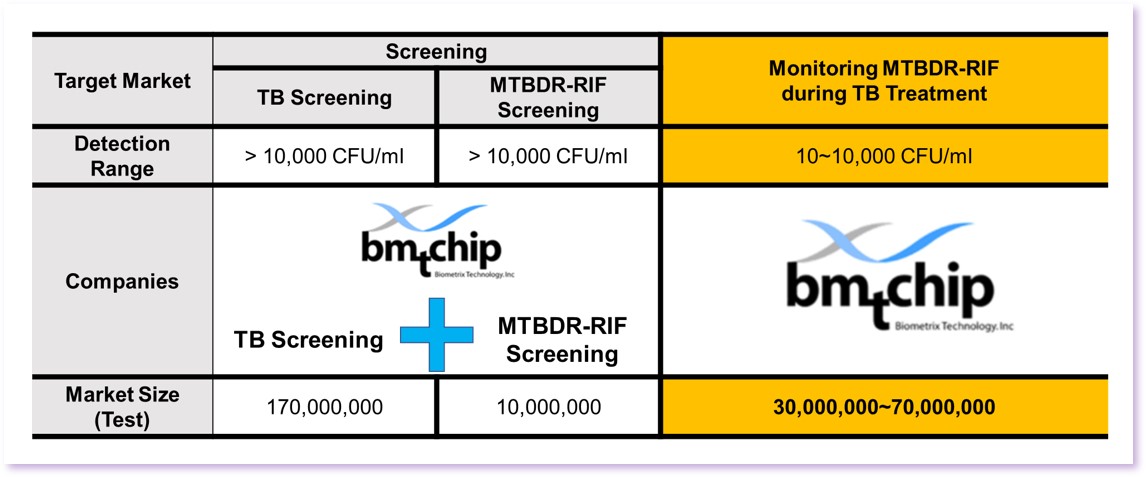
Why can TB and MTBDR-RIF be detected simultaneously when monitoring treatment.
· WHO guidelines recommend cross-validation with more than one test method when monitoring treatment.
· Tuberculosis decreases below 10,000 CFU/ml in 1 to 2 months after starting treatment.
· MTBDR-RIF 9G Membrane KITTM can continuously track up to 10 CFU/ml when monitoring treatment.
· MTBDR-RIF 9G Membrane KITTM is the only ones that can be used cell culture (Drug Susceptible test : DST)
How to use MTBDR-RIF 9G Membrane KITTM
- References
-
- Detection of Multiple Mutations in a Single Codon of Genomic DNA. Chem Commun. 2014, Oct 21;50(82):12344-12347
- Multiplex SNP Detection in Multiple Codons for Accurate Drug Therapy. Chem Commun. 2014, Dec 4;50(93):14585-14588
- MTB-DR-RIF 9G Membrane : A Platform for Multiplex SNP Detection of Multidrug-resistant TB. Anal Bioanal Chem. 2015 Jul;407(19):5739-5745
- MTB-DR-RIF 9G test : Detection and Discrimination of Tuberculosis and Multi-Drug Resistant Tuberculosis Strains. Tuberculosis. 2015 Dec;95(6):780-785
- Accurate Detection of Rifampicin-Resistant Mycobacterium Tuberculosis Strains. Sensors. 2016 Mar 15;16(3):376