본문
Infectious disease test 6HCV Genotyping 9G test™
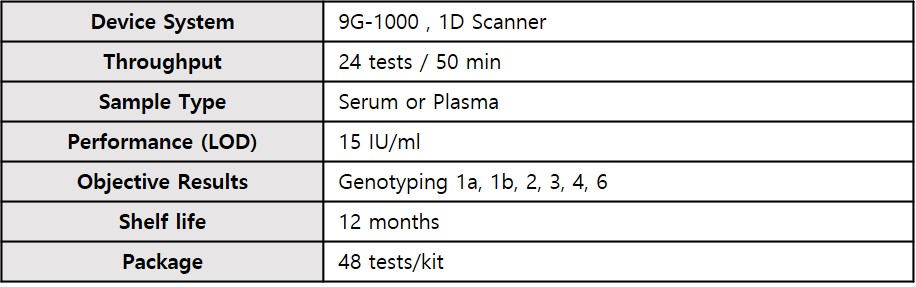
- Sample type
- Serum or Plasma
- Shelf life
- 12 months
- Package
- 48 tests/kit
- Compatible Device
- BMT 9G-1000, BMT 1D scanner
Specification
Detection of 6 HCV subtype using One PCR and Single Set of PCR primers.
· 6 subtypes of Hepatitis C virus (1a, 1b, 2, 3, 4, 6) are detected.
· 6HCV Genotyping 9G test™ detects hepatitis C virus very sensitively(Limit of Detection : 15 IU/ml) and shows sensitivity 99.1% and specificity 99.8%.
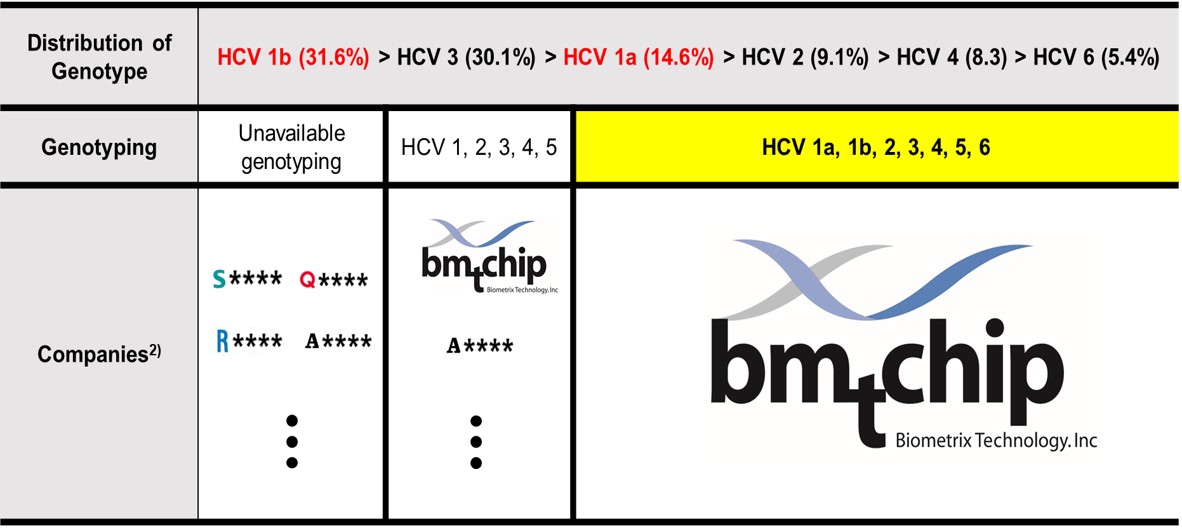
6HCV Genotyping 9G testTM is the only product that can accurately distinguish between HCV 1a, 1b, and 6.
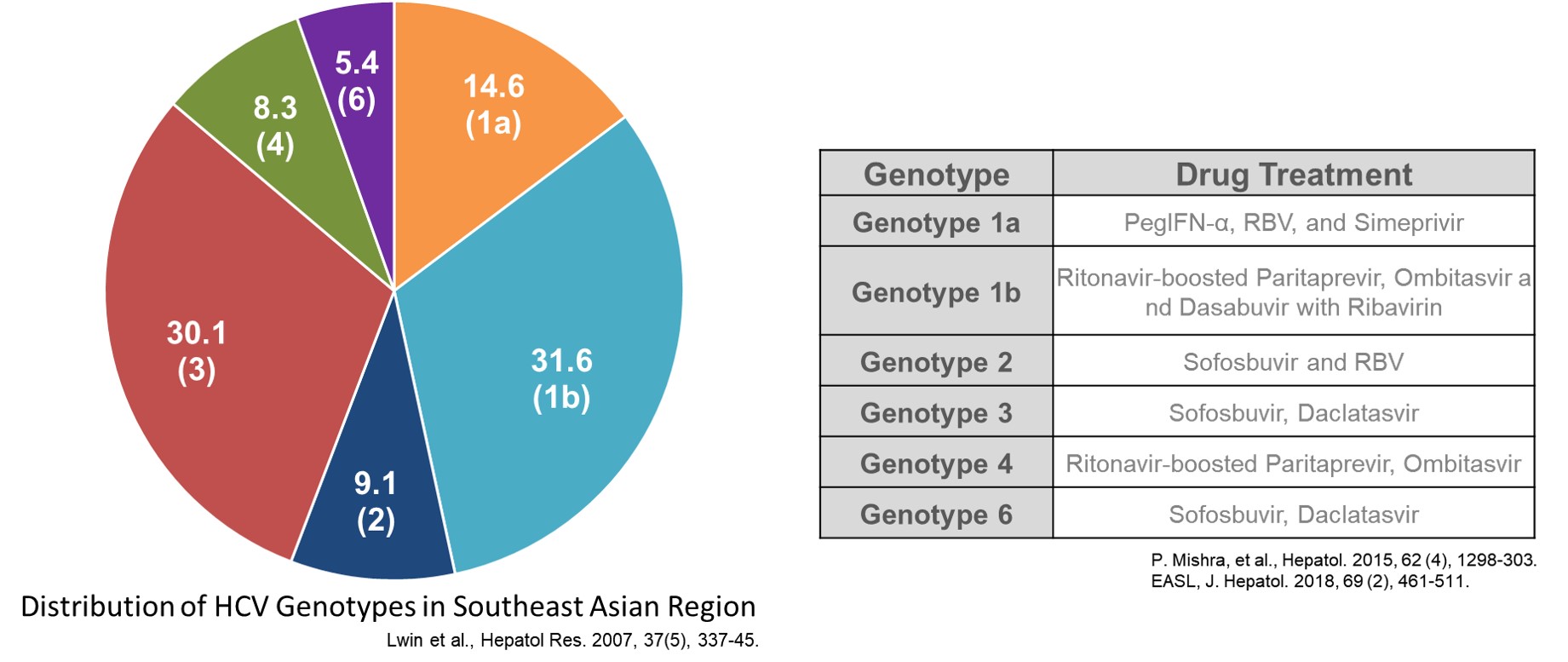
· WHO recommends the identification of HCV genotypes for the choice of the precise treatment regimen.
· Commercial kits cannot genotype HCV 1a, 1b, and 6 correctly. (Asian Pac J Allergy Immunol. 2007, 25, 175~82; PloS One. 2016, 11(4), e0153754; J. Clin. Microbiol. 2014, 52, 3675-3692)
· Only 6HCV Genotyping 9G testTM that applied 9G technology and Single nucleotide polymorphism analysis technology can distinguish between HCV 1a, 1b and 6.
· 6HCV Genotyping 9G testTMcan provide correct drug regimen for the fast cure of HCV patients.
How to use 6HCV Genotyping 9G testTM
- References
-
- Development of a Method for Screening and Genotyping of HCV 1a, 1b, 2, 3, 4, and 6 Genotypes. ACS omega. 2020;5(19):10794-10799
- 6 HCV genotyping 9G test and its comparison with VERSANT HCV genotype 2.0 assay(LiPA) for the hepatitis C virus genotyping. Journal of Virological Method. 2017;239:1~8.
- 6 HCV genotyping 9G test for HCV 1a, 1b, 2, 3, 4 and 6 (6a, 6f, 6i and 6n) with high accuracy. Journal of Virological Method. 2017;246:95~99.
- HCV detection, discrimination, and Genotyping Technologies. Sensors. 2018;18(10):3423
- Performance of 6 HCV Genotyping 9G test for HCV genotyping in clinical samples. Virology Journal. 2018;15(1):107.